Explain Four Different Types of Chemical Reaction With Suitable Examples
The main four types of reactions are direct combination analysis reaction single displacement and double displacement. Types of Chemical Reactions With Examples When you mix chemicals you may get a chemical reaction.
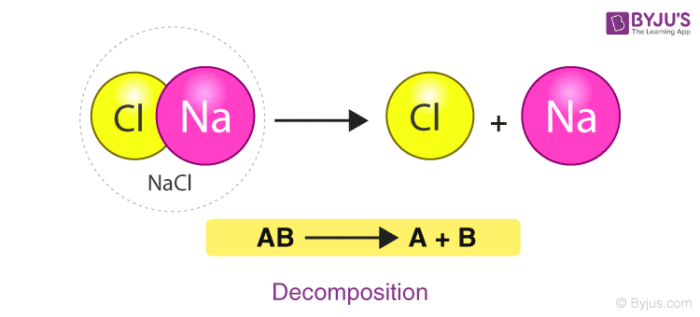
Chemical Reactions Definition Equations Types Examples With Faqs Of Chemical Reactions
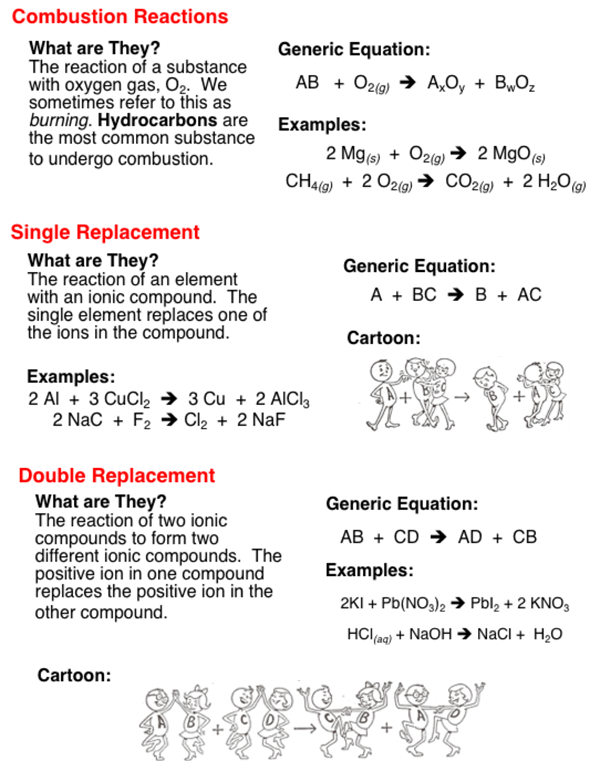
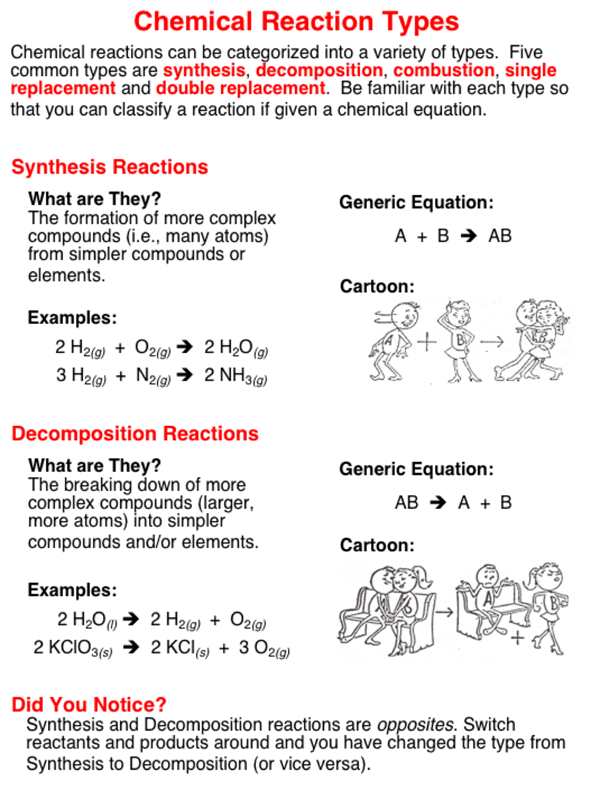
The five basic types of chemical reactions are combination decomposition single-replacement double-replacement and combustion.

. The 5 primary types of chemical reactions are. Examples of organic chemical changes include halogenation methylation crude oil cracking and polymerization. If youre asked the five main types of reactions it is these four and then either acid-base or redox depending who you ask.
As a side note both solvents and catalysts may act as reagents but not as reactants since the. Similar to the previous example H 2 O has a total oxidation state of 0 with each H taking on a 1 state and the O a -2. In a combination reaction two or more elements combine to form a.
Explain the process of corrosion and rusting. Explain four different types of chemical reaction. Different types of reactions are.
Manganese IV oxide. Analyzing the reactants and products of a given reaction. Keep in mind a.
Explain the process of corrosion and rusting. Substances are either chemical elements or. How is exothermic reaction different from an endothermic reaction.
It is a type of continuous ecosystem Reaction. Different types of chemical reaction are. Synthesis reactions happen when two separate atoms or molecules come together to generate a new molecule or substance.
Maximum time- 45 minutes. Chemical reaction a process in which one or more substances the reactants are converted to one or more different substances the products. Because there are so many reactions there are additional ways to categorize them but these other classes will still fall into one of the four main groups.
First we identify four broad classes of reactions based solely on the structural change occurring in the reactant molecules. This classification does not require knowledge or. Substitutions additions eliminations or rearrangements.
For example when barium chloride BaCl 2 and magnesium sulfate MgSO 4 react the SO 4 2 anion switches places with the 2Cl anion giving the compounds BaSO 4 and MgCl 2. Addition reactions elimination reactions substitution reactions pericyclic events rearrangement reactions photochemical reactions and redox reactions are the most common. Types of Chemical Reactions.
Thus decomposition oxidizes oxygen from -2 to 0 and reduces hydrogen. Finally inorganic chemical changes are chemical changes that. The basis for different types of reactions is the product formed the changes that occur the reactants involved and so on.
Oxidation and reduction reaction. How is exothermic reaction different from an endothermic reaction. The reaction in which two or more than two substance combine together to form a single compound.
Three important rearrangement Reactions are 12-rearrangements olefin metathesis and pericyclic Reactions. Learn about the different types of chemical reactions and get. Virtually all organic reactions fall into one of four categories.
2 M g O 2 2. 7 rows Different Types of Chemical Reactions. Types of Chemical Reaction.

Types Of Chemical Reactions Youtube

Types Of Chemical Reactions Detailed Explanation With Example Videos
No comments for "Explain Four Different Types of Chemical Reaction With Suitable Examples"
Post a Comment